Product
PT Borneo Alumina Indonesia
Alumina for National Resilience and Self-Reliance

Alumina, or aluminium oxide, is a white powdery chemical compound with the formula Al₂O₃. It is produced by refining bauxite ore and serves as the primary raw material in aluminium production. Alumina is known for its high melting point, resistance to heat and corrosion, high purity level, and excellent chemical stability.
PT Borneo Alumina Indonesia adopts the Bayer Process as its core technology to produce high-quality alumina. This process has been proven efficient, globally recognized, and environmentally responsible.

How We Refine Bauxite into Alumina
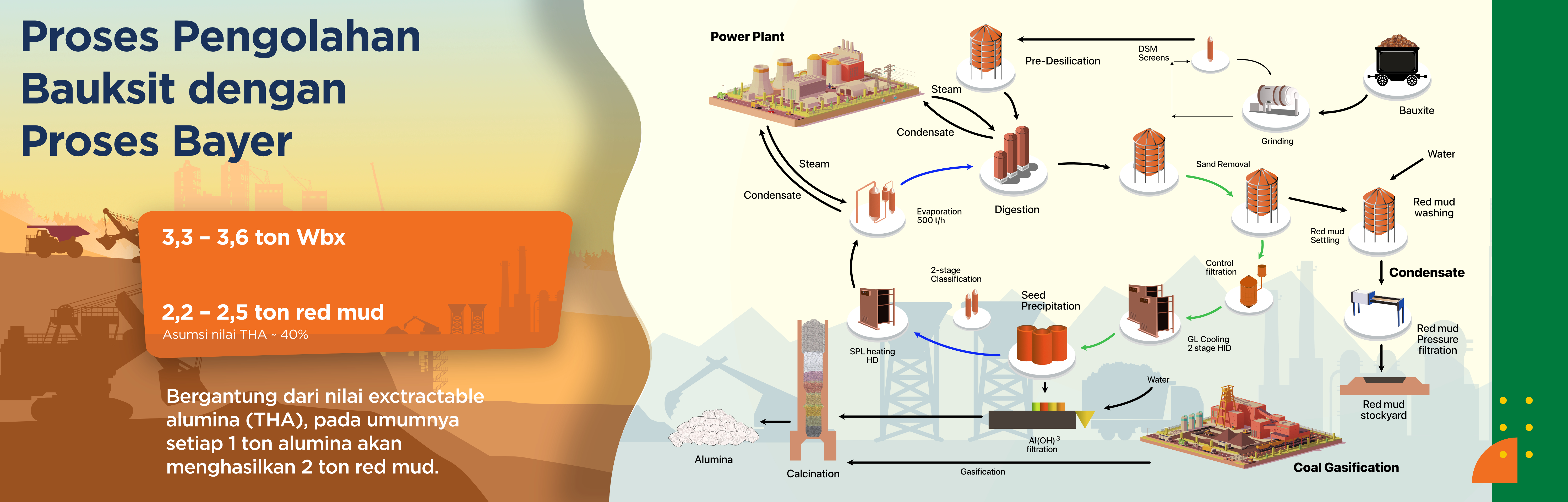
The Bayer Process is the leading and most widely used technology worldwide for refining bauxite into alumina (Al₂O₃), the essential raw material in the production of primary aluminium. Discovered in 1888 by Austrian chemist Karl Bayer, this breakthrough in metallurgy remains highly relevant today due to its efficiency, industrial scalability, and ability to produce high-purity alumina (>98%).
Through a carefully designed series of chemical reactions, the Bayer Process extracts aluminium from bauxite ore in a cost-effective and environmentally responsible manner. The final product is pure alumina, which is further processed through the Hall-Héroult process — a modern electrolysis method that produces the aluminium metal used in a wide range of applications, from aircraft manufacturing to consumer electronics.

Bayer Process Stages
| Digestion: | Crushed and ground bauxite ore is mixed with hot sodium hydroxide (NaOH) solution under pressure in digesters. This reaction dissolves the aluminium compounds to form sodium aluminate, while impurities such as iron and silica remain as solid residues. |
| Clarification: | The digestion mixture undergoes filtration to separate the sodium aluminate solution from the solid residue known as red mud. This step ensures that only aluminium in solution proceeds to the next stage. |
| Precipitation: | The saturated sodium aluminate solution is cooled and seeded with Al(OH)₃ crystals to initiate precipitation. Aluminium hydroxide then settles as a solid. |
| Calcination: | The Al(OH)₃ precipitate is dried and heated (calcined) at approximately 1000–1100°C in calciners, removing water molecules and producing pure alumina (Al₂O₃) in its final form. |
To this day, the Bayer Process remains largely unchanged and continues to be the primary method for producing nearly all of the world’s alumina supply as an intermediate step in aluminium production. Through the implementation of the Bayer Process, PT Borneo Alumina Indonesia is committed to producing high-quality alumina in a sustainable and efficient manner. We believe that with the right technology and responsible operational practices, Indonesia’s alumina industry has the capability to compete globally while contributing positively to national development.